Our amazing grad students Dianise Rodriguez-Garcia & Christina Mecca were featured on a podcast episode at the 2024 USASP conference to discuss what their research focuses on!
Start at 1:36 to hear Dianise talk about the role of skin cells in sickle cell disease pain.
Start at 15:35 to hear Christina talk about the role of skin cells in pain after nerve injury.
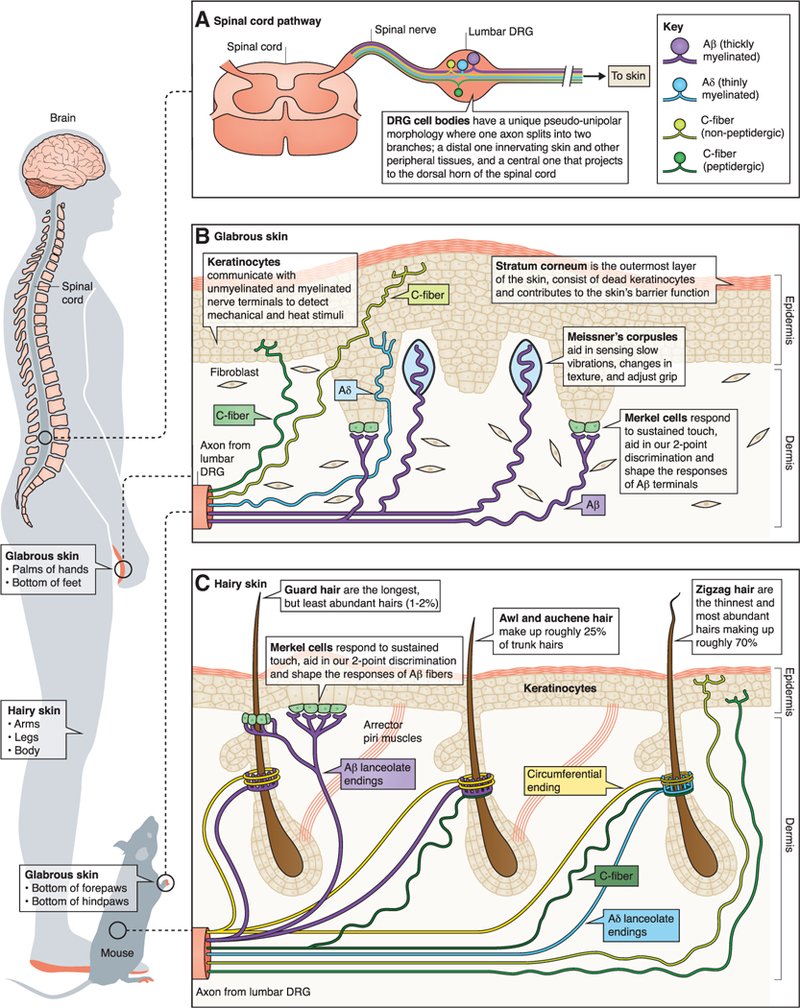
Keratinocyte involvement in somatosensation
Our lab is interested in the bi-directional signaling that occurs between sensory neurons and non-neuronal cells within the epidermis. We are particularly interested in the molecular and cellular mechanisms through which keratinocytes, the most abundant epidermal cell type, relay somatosensory signals to primary sensory afferents. Recent work from our lab demonstrates the necessity of keratinocytes for normal touch sensation; when mechanically stimulated, keratinocyte membranes depolarize leading to ATP release through an uncharacterized mechanism. Released ATP binds to P2X4 receptors on sensory neurons, resulting in neuronal membrane depolarization. These studies are among the first to highlight keratinocytes as critical players in peripheral somatosensory relays and suggest that these cells are more than just an “environmental barrier”. We are continuing to examine how keratinocytes participate in other somatosensory modalities using cutting-edge genetic, electrophysiological, and behavioral approaches. Additionally, we are exploring how keratinocyte-to-sensory neuron signaling is altered in common cutaneous chronic pain conditions including chemotherapy induced and diabetic neuropathies in order to identify non-opioid based topical analgesics.Neural Basis of Sickle Cell Disease Pain
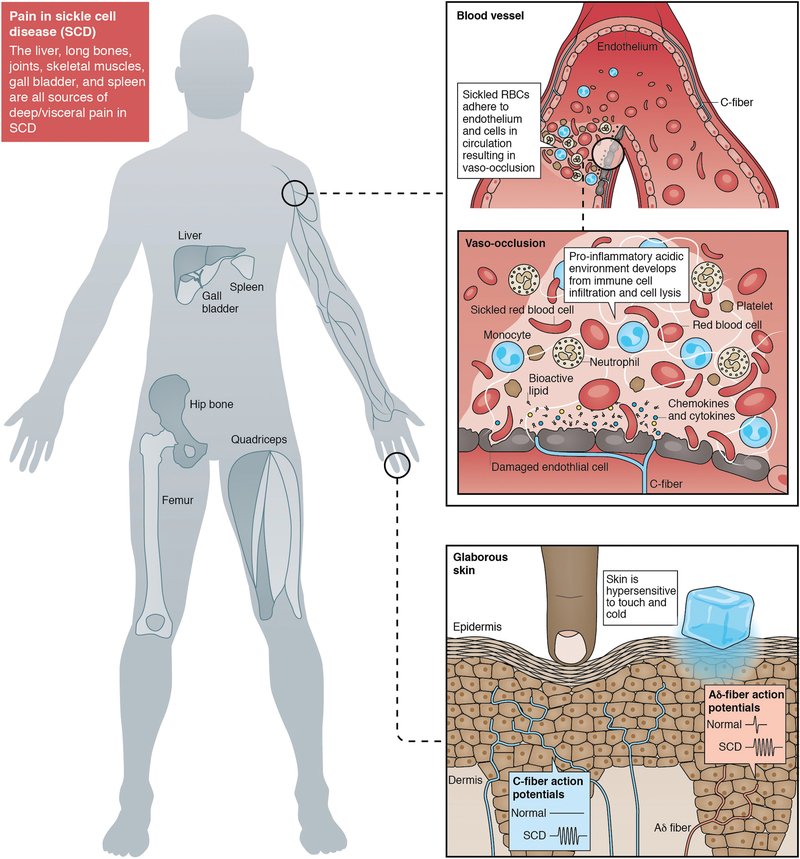
We are one of the few basic science labs examining the neural basis of sickle cell disease (SCD) pain. SCD is the most common genetic blood disorder in the United States, affecting ~100,000 patients. The leading cause of hospitalization for patients with SCD is pain; patients experience debilitating acute pain during vaso-occlusive episodes and >30% of patients develop chronic, non-episode-related pain as their disease progresses. Effective, non-opioid based treatments for SCD pain are lacking. Using two transgenic SCD mouse models, our lab is identifying novel targets for SCD pain management in the peripheral nervous system. We also maintain an extremely productive collaboration with clinical colleagues who regularly treat patients with SCD in order to increase the translatability of our rodent findings. Exploring Pain Mechanisms in Fabry Disease
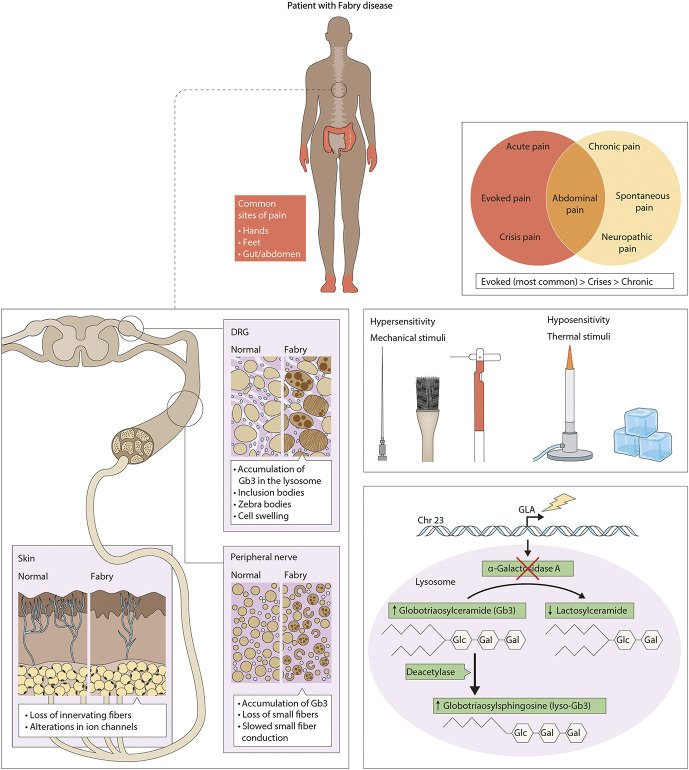
Fabry disease is one of the most common and debilitating lysosomal storage disease. It affects between 1 in 3,000 and 1 in 60,000 people. The condition is a result of a deficiency of a lysosomal enzyme, α-galactosidase A, which leads to the accumulation of glycolipids within the lysosomes of many cell types. Accumulation of these glycolipids has been shown to be present in sensory neurons. These patients consequently experience episodic and chronic pain that is neuropathic in nature. Recently a rat model that exhibits phenotypic and behavioral attributes, similar to those found in patients with the disease, was created at MCW. In collaboration with Dr. Nancy Dahms and Dr. Iris Kassem, our lab uses these Fabry rats as a model for studying the various mechanisms underlying the development and maintenance of the pain associated with this disease.